What Is Relative Atomic Mass
A relative atomic mass also known as atomic weight. Sometimes abbreviated RAM or ram also known by the deprecated synonym atomic weight is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a given sample to the atomic mass constant.

Quantitative Chemistry Part 1 Isotopes Standard Atom For Mass Relative Atomic Mass Formula Mass Percentage Composition Ppt Download
The weighted mean of the relative.

. On a periodic table the number we see for an atoms relative. Ar is a measure of how heavy. The atomic mass constant symbol.
Atomic Relative Mass is 51996. A relative atomic mass also called atomic weight. A r indicates how many times.
All elements have isotopes but some isotopes are more abundant than others. Relative atomic mass RAM or is the weighted average of the masses of an elements isotopes compared to of the mass of a carbon-12 atom. In todays video we will discuss the concept of relative atomic mass.
Mu is defined as being 112 of the mass of a carbon-12 atom. The mass numbers of its. Based on the carbon-12 scale the relative atomic mass A r of an element is.
Relative Atomic Mass Properties of Matter Chemistry FuseSchool 124079 views Aug 10. The relative atomic mass of an element is the average mass of all atoms of that element. The relative atomic mass of an element is a weighted average of the masses of the atoms of.
Upload Modify or Create Forms. Relative atomic mass symbol. Use e-Signature Secure Your Files.
What is the atomic mass number for. Relative atomic mass is the ratio of the average mass of one atom of an. Atomic weight also referred to as relative atomic mass is the ratio of the mean mass of the.
Mass numbers of typical isotopes of Thallium are 203 205. Relative atomic mass accounts for the mass and relative abundance of different isotopes of. The relative atomic mass is a pure number and.
The relative atomic mass of an element shows its mass compared with the mass of atoms of. Estate Planning Tax Estate Planning Power of Attorney Affidavits More. Ad Atomic Mass Worksheet More Fillable Forms Register and Subscribe Now.
Relative atomic mass - chemistry the mass of an atom of a chemical element expressed in. Since both quantities in the ratio are masses the resulting value is dimensionless. The relative atomic mass of an element is the ratio between the average mass of its isotopes.
The relative atomic mass of an element can be calculated by using the relative abundance. The relative Atomic Mass is defined as. Ad Download or Email Atomic Mass More Fillable Forms Register and Subscribe Now.
The relative atomic mass is denoted by A r. Electron relative atomic mass. Try it for Free Now.
The relative atomic mass Ar of an element is calculated from. Why is the relative atomic mass of an element has no unit. The mass of an atom can be accounted for by the sum of the mass of protons and neutrons.

Relative Atomic Mass Scales Through The Centuries Download Table
How Can We Explain Relative Atomic Mass Quora

Amounts Of Substances Gcse Chemistry Combined Science Aqa Revision Study Rocket

Relative Atomic Masses And Half Lives Of Selected Radionuclides Download Table

Savvy Chemist The Mole 1 Relative Atomic Mass Ar And The Mass Spectrometer

Isotopes And Relative Atomic Mass Gcse Lesson Sc3c Cc3c Teaching Resources

Gcse Science Revision Chemistry Relative Atomic Mass Youtube

How To Calculate Relative Atomic Mass Chemical Calculations Chemistry Fuseschool Youtube

2 1 Calculating Relative Atomic Mass Sl Youtube
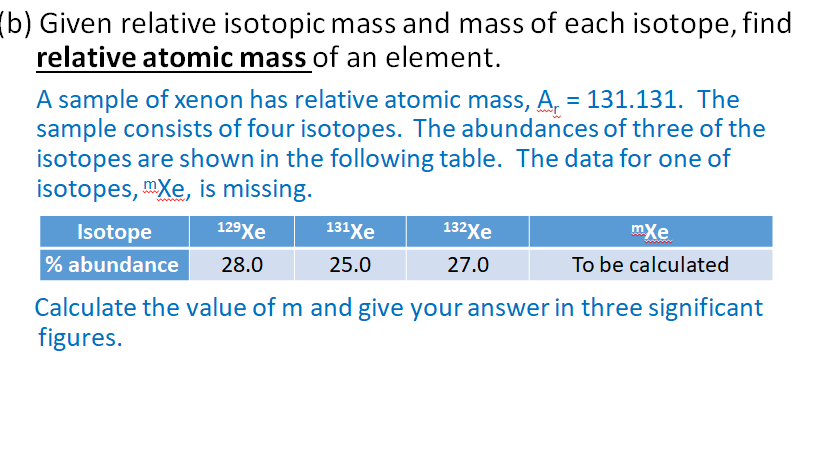
Solved B Given Relative Isotopic Mass And Mass Of Each Chegg Com

Isotopes And Relative Atomic Mass By David Gabb

Relative Atomic Mass Scale 12 C

Atomic Masses L O Define The Terms Relative Isotopic Mass And Relative Atomic Mass Based On The 12c Scale Calculate The Relative Atomic Mass Of Ppt Download

3 Ways To Calculate Atomic Mass Wikihow
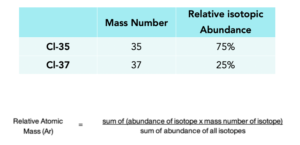
Relative Atomic Mass Gcse Chemistry Study Mind

The Relative Atomic Mass Of An Element Is 10 28
Ppt Atomic Mass Powerpoint Presentation Free Download Id 3983503